
Interview with Laia Movilla, Product Specialist at Sherpapharma
Today we interview Laia Movilla, the Product Specialist at Sherpapharma. Laia has a Bachelor’s degree in Biomedical Engineering from the Universitat Politècnica de Catalunya. At Sherpapharma, she is in charge of defining system requirements, validating new developments, and providing customer support.
Hereunder, she is going to explain the key points that make Sherpapharma the best environmental monitoring software.
1. Tell us about your beginnings at Tiselab.
I started at Tiselab in 2018 on an internship while in my last year of college. From the very start, the company placed a lot of trust in me which enabled me to learn a lot. After going to Italy on an Erasmus to write my thesis and obtaining my degree, I started working here full-time.
2. What is your day-to-day life at the company like nowadays?
I work with a team and we’re lucky to participate in most of the software development cycle. One of our main tasks is to collect client’s requirements and feedback and transfer them, together with our own requirements, to the development team so they can implement them. Then we make sure all the new functionalities are working correctly and are fully data integrity compliant. Once a new version is released, we perform a full GMP validation which is delivered to all of our customers. We also take care of client support which ranges from helping with the set up of new clients to solving any doubts they may have when using SherpaPharma.
3. Could you describe SherpaPharma in four lines?
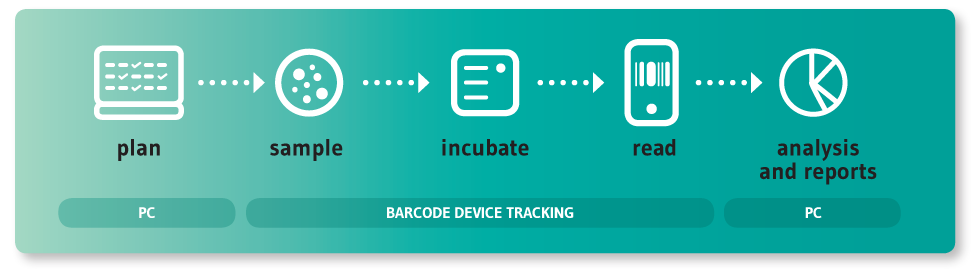
SherpaPharma is an innovative solution that allows pharmaceutical companies to have complete control over their environmental monitoring activities by simplifying the scheduling process and by recording all data and metadata necessary to generate reports and data analysis.
4. Let’s start with cleanrooms. What shortcomings did you detect in cleanrooms in terms of environmental monitoring before implementing SherpaPharma?
When companies approach SherpaPharma their main weak points are their difficulty to keep track in real-time of all the monitoring activities that are being performed in their premises since everything is written on paper and, additionally, the amount of time that is needed to generate comprehensible reports to analyse their data in case of an investigation.
5. What does SherpaPharma bring to cleanrooms in terms of time, and can you give us an example?
SherpaPharma is undeniably a time-saving tool. This can be seen in all of the stages of the environmental monitoring process. Sampling events can be planned with ease to meet the companies’ SOP. The use of a hand-held device enables any user to record a sampling instance by solely scanning the sampling position and the culture media, while doing so the user can also add any additional information about the activity. Furthermore, an in-depth analysis of the recorded data is available at the click of a button.
6. What are the risks of an incorrect environmental monitoring process in a cleanroom? Could you give us an example?
Like with any process in the pharmaceutical world, an incorrect monitoring process can result in a loss of product quality or even put patient’s health at risk. As an example, if a company is unable to react quickly to an out-of-specifications result because the root-cause analysis took too long and the source is problematic enough to stop production, multiple batches could be under investigation or the release process could be delayed.
7. How does SherpaPharma avoid these risks?
When using SherpaPharma, all the metadata associated with any action performed is recorded. This enables the users to easily see the information related to each sampling instance. Also, when introducing a new reading the system instantly alerts the user if it is an out-of-specifications. On top of that, the system offers different options to instantly analyse the introduced data and obtain reports from it.
8. Let’s now look at environmental monitoring from the laboratory’s point of view. What shortcomings do you detect in the microbiology laboratory before implementing SherpaPharma?
The main issue that laboratories have is the amount of time invested in transcribing the monitoring data into an Excel. Moreover, this procedure does not ensure data integrity which can lead to erroneous data analysis.
9. Is SherpaPharma capable of optimising the time of those working in the laboratory? How?
By using SherpaPharma users are not only saving a huge amount of time by removing the need for manual data transfer or report generation but also guarantees full data integrity.
10. What are the main risks involved in an incorrect environmental monitoring process in the laboratory? Could you give us an example?
Because of the manual aspect of the recording process, human-related errors can happen. This, in its turn, can cause an inaccurate interpretation of the monitoring results which may result in an incorrect assessment of the risk.
11. How does SherpaPharma combat these errors?
The way SherpaPharma avoids this kind of mistakes is by being 100% data integrity compliant.
12. What does 100% compliance with data integrity imply?
A full compliance with data integrity implies three main topics. The first one is the fact that any metadata introduced into the system cannot be modified in order to preserve the original record. The second one involves asking for a reason and signature to justify any data modification. Finally, any change done in the system is tracked by a non-editable Audit Trail.
13. What is the process to implement SherpaPharma in the company? What are the steps?
After a practical demo of the system functionalities, we evaluate the customer’s requirements and how suitable SherpaPharma is to their current process. Once the contract is signed and data confidentiality is ensured, we immediately start the transition phase where the SOPs are analysed and introduced into a test server. In this environment, the client can check if the proposed organisation is the most convenient for their daily operations. Then all future users are trained. When the testing phase finishes, all data is migrated into a GMP environment where the client can start recording all their environmental monitoring data using only SherpaPharma.
14. What devices are necessary for environmental monitoring with SherpaPharma?
The data entry performed in the cleanrooms can be done with a hand-held barcode reader similar to a smartphone. This device, which can be disinfected, instantly transfers all monitoring data to SherpaPharma allowing all records to be checked from any computer. An NFC wristband can also be used to enter the user’s email into the device, then the only thing they have to do to log in is introduce their password.
15. What is the opinion of those who already work with SherpaPharma?
Our customers are very satisfied with the amount of time that they are saving by using the software. Everybody also remarks that thanks to SherpaPharma they have higher control over their environmental monitoring process and highlight the system’s reliability and effectiveness.
***
¿Do you want to know more about SherpaPharma? Contact us >